Introduction: Daratumumab (dara) and hyaluronidase-fihj was FDA approved for the treatment of newly diagnosed (NDMM) and relapsed/refractory multiple myeloma (RRMM) in May 2020, which offers a novel subcutaneous (SQ) administration for dara. The COLUMBA trial investigated the efficacy and safety of SQ dara (n=263) versus intravenous (IV) dara (n=259) in RRMM patients. The ORR was 41% with SQ dara versus 37% with IV dara (RR 1.11, 95% CI: 0.89-1.37). All-grade infusion-related reactions (IRR) were lower at 13% with SQ dara compared to 34% with IV dara. Other grade 3/4 adverse events were similar across both groups, with neutropenia being slightly higher with SQ (13% vs 8%). SQ dara was non-inferior to IV dara in terms of efficacy and offers MM patients a quicker administration with less risk for reactions. Since this approval, we have implemented all new patients to receive dara SQ and have transitioned IV dara patients to receive SQ formulation in efforts to improvise patient safety, without losing the efficacy. Additional advantages of reduced chair time and patient convenience made this an appealing option. The relative effect of patient satisfaction with this transition is unknown.
Methods: We identified MM patients who received dara off-trial across our institution, which encompasses 150 infusion chairs across five ambulatory infusion centers, from June 1, 2020 through July 29, 2020. All patients on IV dara were switched to SQ for their next cycle after being seen at their regularly scheduled clinic visit where the change was discussed. SQ dara was administered via a 5-min SQ push and no observation period was implemented for patients who had previously received IV dara. A 3.5 hour observation was only utilized for cycle 1, day 1 SQ dara patients, based on the median time to IRR on COLUMBA trial. We analyzed the number of SQ and IV doses dispensed on a daily basis in order to capture the overall adoption rate of the SQ formulation. We also aimed to have our organization model a quick and safe transition between formulations for similarly sized cancer centers, Further, a five question patient satisfaction questionnaire was developed to administer to patients who switched from IV to SQ dara to assess their satisfaction with the new product.
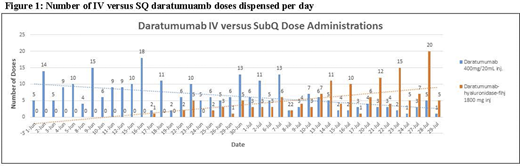
Results: From June 1 through July 29, 2020, a total of 417 doses of daratumumab were dispensed across all facilities at our intuition. The first patient treated with SQ dara was on June 17 after CPOE orderset development and staff education were completed. The number of SQ versus IV doses dispensed per day are displayed in Figure 1 and demonstrate the decrease in IV doses and subsequent increase in SQ doses over time. The adoption rate for the month of June was 90.5% IV dara and 9.5% SQ dara. Through July 29, the adoption rate shifted to 39.9% IV dara and 60.1% SQ dara. The patient questionnaire is ongoing and results will be presented at the time of the meeting. The economic implication of this conversion has resulted in 300 hours of reduced infusion chair usage. Updated formulary transition data will also be presented at the time of the meeting, as the formulary switch is ongoing.
Conclusion: Our current adoption rate of 60.1% highlights the success of a large, academic medical institution in switching patients to the novel SQ dara formulation over a 6-week period from the first SQ patient. Our strategy of switching on a rolling basis allowed for patients to be counseled by a PharmD on this upcoming change. The daily dispense report assisted with drug ordering to ensure significant cost savings by reducing our purchases of IV dara and confirming adequate supply of SQ dara. Our goal is greater than 95% conversion to the SQ formulation, with minimal IV use reserved for patient preference or uncommon clinical needs. The patient satisfaction questionnaire results will inform providers of any concerns with this change. The implementation of SQ dara has streamlined drug administration and allowed for an increase in infusion-chair availability.
Maples:The Lynx Group LLC: Consultancy; GlaxoSmithKline: Consultancy. Kaufman:Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Tecnopharma: Consultancy, Honoraria; Sanofi/Genyzme: Consultancy, Honoraria; Karyopharm: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; AbbVie: Consultancy. Hofmeister:Janssen: Honoraria, Research Funding; Oncolytics Biotech: Research Funding; Oncopeptides: Honoraria; Nektar: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Imbrium: Honoraria; Sanofi: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding. Dhodapkar:Lava Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other; Amgen: Membership on an entity's Board of Directors or advisory committees, Other; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Other; Janssen: Membership on an entity's Board of Directors or advisory committees, Other; Kite: Membership on an entity's Board of Directors or advisory committees, Other; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other. Lonial:Merck: Consultancy, Honoraria, Other: Personal fees; Millennium: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Other: Personal fees, Research Funding; JUNO Therapeutics: Consultancy; Abbvie: Consultancy; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Karyopharm: Consultancy; Sanofi: Consultancy; Onyx: Honoraria; Amgen: Consultancy, Honoraria, Other: Personal fees; Takeda: Consultancy, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; GSK: Consultancy, Honoraria, Other: Personal fees; Janssen: Consultancy, Honoraria, Other: Personal fees, Research Funding. Nooka:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Technologies: Consultancy, Honoraria; Spectrum Pharmaceuticals: Consultancy; Oncopeptides: Consultancy, Honoraria; Karyopharm Therapeutics, Adaptive technologies: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Other: Personal Fees: Travel/accomodations/expenses, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding.
Daratumumab and hyaluronidase-fihj was approved for the treatment of multiple myeloma in specific combinations. Our abstract describes the conversion from daratumumab to daratumumab and hyaluronidase-fihj for all patients which will include some off-label combinations.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal